The DEPTH Framework: A Proven Roadmap for Rolling Out PharmChek® Sweat Patches

August 4, 2025
Traditional drug testing is invasive, logistically draining, and easily manipulated.
Urine testing interrupts daily schedules, requires same-gender observation, and only captures a snapshot in time. For courts and treatment teams striving to build accountability and support long-term behavior change, point-in-time results aren't enough.
The PharmChek® Sweat Patch offers a better path: continuous, tamper-evident drug detection over 7 to 10 days. But like any tool with high forensic value, it needs thoughtful implementation. That’s where the DEPTH Framework comes in—a proven PharmChek® rollout roadmap developed by practitioners for practitioners.
Who This Guide Is For: Treatment court coordinators, program directors, probation chiefs, clinicians, and anyone responsible for drug monitoring in recovery and justice settings.
Key Takeaways
What is the DEPTH Framework?
The DEPTH Framework is a five-step rollout model: Do the Math, Educate, Plan the Roll-Out, Train, and Hold the Line. It was developed to help agencies implement the PharmChek® Sweat Patch effectively and sustainably.
Why is a rollout framework needed?
Sweat patch programs can fail without early buy-in, proper training, or clear policy. The DEPTH model prevents missteps that can erode trust and program outcomes.
Who created it?
Juvenile Court Director Ed Gilligan developed the framework based on firsthand lessons from a failed rollout and a successful second attempt in Yuma County.
What are the benefits of using DEPTH?
Agencies using the DEPTH Framework report stronger stakeholder trust, better participant outcomes, and fewer logistical surprises, while protecting the patch’s forensic value.
Where can I find support materials?
The article includes a training checklist, implementation FAQ, and cost comparison chart to help teams launch with confidence.
What Is the DEPTH Framework?
The DEPTH Framework was born from experience, not theory. Juvenile Court Director Ed Gilligan created the acronym after experiencing the consequences of a previously poorly planned PharmChek® rollout that lacked structure, strategy, and stakeholder buy-in. Officers received minimal training, patches sat unused, and cases got messy. And after it failed, Gilligan realized his missing piece: depth.
Gilligan's revised approach to rolling out PharmChek® formed the basis of the DEPTH framework:
- D (Do the Math): Budget, cost-compare, and plan for sustainability
- E (Educate): Secure broad stakeholder understanding and trust
- P (Plan the Roll-Out): Select pilot cohorts and define implementation rules
- T (Train): Certify applicators and build system-wide comfort
- H (Hold the Line): Maintain fidelity even under pressure
The DEPTH Framework minimizes resistance, maximizes adoption, and keeps programs from reverting to reactive urine testing. Whether you're preparing for a pilot or building a program-wide model, DEPTH offers a defensible, adaptable roadmap.
At the end of this article, we’ll present a bonus letter in the DEPTH framework—a step that has proven extremely valuable to Gilligan and his team.
Why Use the PharmChek® Sweat Patch?
Continuous Monitoring and Deterrence
Traditional drug testing leaves large gaps between observed tests. Participants learn quickly how to “beat” urine tests by timing use or diluting samples. The PharmChek® Sweat Patch fills those gaps. Worn for an average of 10 days, it continuously collects the contaminants of drugs in insensible perspiration and provides a full wear-time detection window, not just a moment in time.
This creates a deterrent effect. Participants know they can't outmaneuver the patch. According to Judge Greg Pinski, it also builds therapeutic leverage:
"It serves a complementary purpose to any drug testing program... It's accurate, it's valid, and it's withstood scrutiny."
Tamper Resistance
Urine can be diluted. Oral fluid can be contaminated. But PharmChek® is tamper-evident. If removed or altered, the patch shows visible signs. When paired with a photo documentation protocol, covered in more detail below, even low-level tampering can be flagged and addressed without the ambiguity that often clouds urinalysis disputes.
Detection Window Advantage
The patch can detect use that occurred:
- 24–48 hours before application (the transdermal delay of drugs breaking down in the body and being excreted in sweat)
- Any time during the wear period
- 24 hours prior to removal
Compare that to the narrow 24–72 hour detection window of urine, which can easily be missed with timing, hydration, or substitution.
Comparison Chart: PharmChek® Sweat Patch vs. Urine vs. Oral Fluid
| Feature | PharmChek® Sweat Patch | Urine Testing | Oral Fluid Testing |
|---|---|---|---|
| Detection Window | 24–48 hrs prior + 7–10 days | 1–3 days per test | 12–48 hours |
| Frequency Needed | 1 patch/week | 2–3 collections/week | Daily or near-daily |
| Tamper Evidence | High (visible, adhesive integrity, photo protocol) | Low to Moderate (observed collection required) | Moderate (easy to adulterate with food/drink) |
| Collection Supervision | Trained Observer | Requires supervision | Trained Observer |
| Chain of Custody | Continuous via sealed patch + photos | Depends on staff + sample handling | Time-sensitive; less stable |
| Drug Classes Detected | Parent drug + metabolite (methamphetamine & cocaine) | Typically metabolites only | Mostly parent drugs |
| Passive Exposure Defense | Parent drug + metabolite requirement ensures ingestion. | Weak (may detect only metabolites) | Weak (no metabolite confirmation) |
| Ideal Use Cases | Tests going forward: Trauma-informed collection, long-term monitoring, behavior change & relapse prevention, reward or sanction framework, high-risk/tamper-prone individuals | Tests looking back: Routine or short-term monitoring | Tests looking back: Immediate detection, post-incident use |
Source: PharmChek® Technical Guide, 2024
Step-by-Step Rollout Guide Using DEPTH
The DEPTH Framework was designed to be more than a list of best practices. It provides structure to your rollout process and ensures consistency across teams, agencies, and participant experiences. Each stage (Do the Math, Educate, Plan, Train, and Hold the Line) plays a critical role in building a sustainable, defensible drug testing program. Below, we walk through each step, with insights from real-world implementations and lessons learned.
Do the Math: Know Your Budget, Savings, and Risk
Before launching PharmChek®, understand the numbers:
- Current cost of 3 urine tests per week per participant
- Cost of confirmation testing for presumptive positives (LC-MS/MS)
- Rate of diluted or missed UAs (and cost of retesting)
- Available revert-back funds or grants
- Annual cost of sweat patch testing (per participant x lab fees)
In his program, Ed used end-of-year revert-back funds to pay for patches in Yuma County:
“We seize that money at year's end and we buy all of the product and prepay for all of the lab fees for the next year.”
This allowed his team to secure a full year’s worth of sweat patch supplies and lab services, ensuring sustainability without requiring new budget allocations.
Sample Inputs:
Estimates based on industry averages. Actual pricing may vary by region, vendor, and lab service contracts.
- UA cost per test (e.g., $20–$50)
- LC-MS/MS confirmation (e.g., $50+)
- Sweat patch cost (e.g., $40–$60 per patch + lab)
- Staff time savings by reducing observed collection
Sample Comparison Table: 3 UAs/week vs. 1 Patch/week
| Cost Component | 3 Urine Tests/Week | 1 Sweat Patch/10+ Days |
|---|---|---|
| Number of Tests per Month | ~12 | 4 |
| Average Cost per Test | $20–$50 | $40–$60 |
| Monthly Testing Cost | $240–$600 | $160–$240 |
| Observation Staff Time | High (3x/week supervision) | Low (apply & remove every 10 days) |
| Confirmation Testing (LC-MS/MS) | Typically an additional cost | Automatic confirmation by LC-MS/MS |
| Tamper Detection | Moderate | High |
| Missed Appointment Risk | Higher | Lower |
| Total Monthly Burden | Higher logistical/admin load | Streamlined process |
Based on industry averages. Actual costs may vary depending on vendor agreements and jurisdiction size.
Educate: Stakeholder Buy-In Is Non-Negotiable
Sweat patches differ from urine tests, and a misunderstanding can lead to resistance or misapplication.
Ed Gilligan cautions:
“By the time we got out to the judge and the treatment provider, they weren’t involved... and it hurt us long-term.”
Gilligan emphasizes the need to educate all parties involved:
- Judges: Explain confirmation science and how to interpret time frames
- Attorneys: Share case law and FDA clearance
- Clinicians: Reinforce trauma-informed alternatives to observed UAs
- Participants: Explain the detection window and tamper policies
In Yuma County, for example, Ed Gilligan emphasized the importance of visual and hands-on learning for his team, particularly after his first rollout attempt failed due to a lack of engagement. His team found that providing repeated exposure to patch application through video tutorials and in-person demos led to greater consistency and fewer handling errors. Consider supplementing online resources with local champions who can reinforce best practices in real time.
Plan the Roll-Out: Start Small, Think Long-Term
Don’t start with your entire caseload. Choose a manageable pilot cohort:
- High-risk youth
- Tamper-prone clients
- Participants with chronic diluted or missed UAs
Create a transparent eligibility matrix. Define how patches will be assigned and how violations will be addressed.
“We selected the successful kids,” said Gilligan, referring to participants who had been showing promising engagement and compliance in other areas of the program. "Out of our first 10 tests, we had two negatives, five positives for THC, and two for methamphetamine. It was an eye-opener. These were not individuals we suspected of current use, and yet the patch revealed a very different story. It confirmed for us that PharmChek® was capturing real-time drug use we would have missed with urine testing alone.”
Decision Tree: Patch Eligibility
Use this sample decision tree to identify when to assign the PharmChek® Sweat Patch:
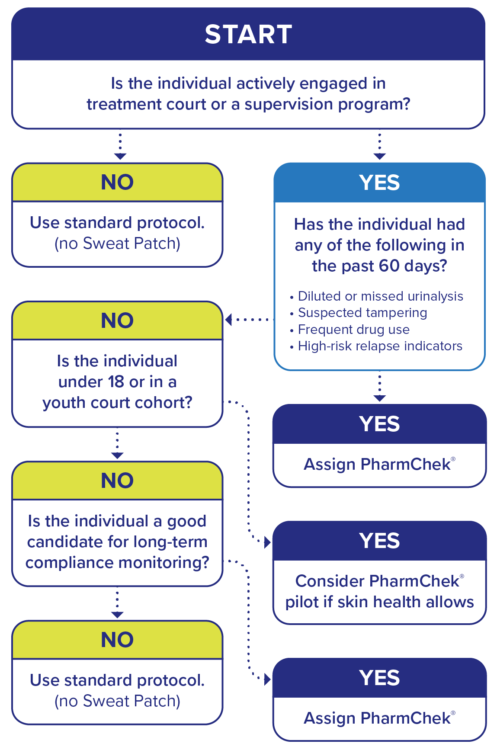
NOTE: Patch assignment should be documented in case notes and reviewed with the team at staffing.
Train: Make Application and Removal Routine
Use our online training and certification to certify PharmChek® applicators, and schedule periodic retraining for consistency.
Topics to cover:
- Rotation of patch application sites
- Common reasons for premature detachment
- How to reapply and document tampering
Hold the Line: Don’t Revert When Results Are Uncomfortable
When your most promising participant tests positive, it’s tempting to question the patch.
“The judge said, 'Let’s get a urine to compare it to the patch.' That’s impossible—they’re different time windows,” Gilligan explained.
When facing pressure:
- Revisit the science (confirmation via LC-MS/MS)
- Share case law or connect with another jurisdiction
- Educate individually rather than backtracking
Photographs: Document Everything
Although photo documentation was mentioned earlier under staff training, it deserves additional emphasis as a best practice that supports both program integrity and courtroom success. Consider it a “bonus” step in the DEPTH protocol, as it can significantly enhance accountability and defensibility.
Take:
- A photo at the time of application (showing intact seal and placement)
- A photo prior to patch removal (showing the condition of the patch and possible tampering)
Benefits:
- Courtroom defensibility
- Chain-of-custody integrity
- Training tool for new staff
Keep a binder of anonymized photos as a training manual and documentation source.
Programs that include photo documentation consistently report smoother staff onboarding, fewer disputes, and stronger evidence in court. For this reason, many programs consider it an essential best practice, even if it's not formally part of DEPTH.
Building Trust Through Science
LC-MS/MS Confirmation
All positive screens are automatically sent for confirmation via LC-MS/MS, the platinum standard in forensic toxicology. This eliminates false positives and ensures results are precise and defensible.
Understand the Role of LC-MS/MS
Discover the risks of inaccurate drug screening and how LC-MS/MS confirmation improves accuracy and reliability.
Cutoff Levels and Sensitivity
Cutoff levels are the established thresholds at which a test result is considered positive. They help labs distinguish between actual drug use and trace, irrelevant exposure. In the context of the PharmChek® Sweat Patch, every presumptive positive is automatically confirmed using LC-MS/MS at scientifically validated cutoff levels, which are often lower than those in urine due to the different composition of sweat.
Examples:
- Methamphetamine: 10 ng/mL (96% sensitivity)
- THC: 0.5 ng/mL (confirmation cutoff)
- Fentanyl: 1 ng/mL
What makes these cutoffs so reliable is that a PharmChek® confirmation requires both the parent drug and its metabolite to be present above the limit of quantitation (meth and cocaine). This two-part requirement ensures ingestion, rather than passive exposure. It also means that, unlike urine, which often detects only metabolites, the sweat patch can identify both the drug itself and the body's processing of it.
Why Do Cutoff Levels Matter?
Learn how drug test cutoff levels work and how they affect outcomes in treatment courts.
Addressing Passive Exposure Myths
PharmChek®’s semi-permeable membrane is specifically designed to block outside contaminants. It prevents direct environmental exposure from compromising the absorbent pad inside the patch. This design has been rigorously tested in controlled studies simulating worst-case environmental exposure scenarios.
To trigger a confirmed positive with the PharmChek® Sweat Patch, both the parent drug and its metabolite must be present above defined cutoff thresholds. This two-part requirement means passive exposure—such as being in a room with secondhand smoke—will not result in a positive test. According to PharmChek®’s technical documentation, external application of drugs to the outside of the patch, even under unrealistic conditions, fails to penetrate the protective polyurethane layer and influence lab results.
“We tested the patch against extreme exposures,” said Dr. Rana during a recent PharmChek® Academy session. “Even unrealistic conditions failed to meet confirmation criteria.”
In one such study, patches were subjected to high concentrations of drug vapor and even direct application to the external film. Only by deliberately damaging the membrane with solvents did researchers observe any impact, and even then, it did not replicate real-world conditions. This demonstrates that a confirmed positive test is extremely reliable evidence of ingestion, not mere proximity.
The requirement for both parent drug and metabolite further strengthens this. Metabolites are only produced after the drug has been processed by the body. They cannot appear in sweat unless the person has used the substance.
Legal and Forensic Credibility
PharmChek® is FDA-cleared—a designation that is not easily achieved. Devices seeking FDA clearance must undergo rigorous validation to demonstrate safety, reliability, and scientific integrity. This process includes laboratory testing, manufacturing quality control, and scientific review to ensure the device meets strict regulatory criteria.
Very few alternative drug-testing devices in the forensic or criminal justice space hold FDA clearance. This makes PharmChek® a standout choice for programs that need a scientifically defensible and court-admissible solution. Courts across the country have cited FDA clearance as a key factor in admitting sweat patch evidence, viewing it as an objective endorsement of reliability.
This status also ensures alignment with other federal standards, including those from SAMHSA and the Department of Transportation, reinforcing its utility in justice, clinical, and safety-sensitive settings.
Dr. Suman Rana emphasized:
“Courts rely on that FDA clearance. It makes the patch easier to admit.”
Frequently Asked Questions (FAQ)
How does the PharmChek® Sweat Patch work?
It collects insensible sweat over days and stores drug evidence in a sealed absorbent pad.
What is the DEPTH Framework?
A rollout roadmap: Do the Math, Educate, Plan, Train, Hold the Line, and Photograph.
Why use a sweat patch instead of urine?
Longer detection, less supervision, tamper-evident, continuous results.
How long can it detect drug use?
PharmChek® can detect use 24–48 hours before application and any use during the 7–10 day wear time.
What if a sweat patch falls off?
PharmChek® does not fall off. Train your staff on reapplication, photo documentation, and assessing tampering.
What drugs are detected?
Standard panels include meth, cocaine, fentanyl, opioids, THC, and more.
Is it court-admissible?
Yes. It is FDA-cleared and upheld in multiple legal challenges.
Do we need an MRO?
No, MROs are not required in criminal justice settings.
90-Day Rollout Checklist
To help your team move from planning to execution, here’s a practical 90-day implementation checklist based on lessons learned from real-world program rollouts:
- Conduct budget analysis and identify funding
- Select pilot cohort
- Train staff via PharmChek® Academy
- Schedule stakeholder education meetings
- Define eligibility rules and patch policies
- Set up a photograph documentation system
- Begin pilot with 5–10 participants
- Collect outcome data (positives, reapplications, compliance)
- Evaluate stakeholder feedback
- Plan scale-up
Putting DEPTH into Practice
The PharmChek® Sweat Patch is a powerful tool, but it’s only as strong as your implementation. The DEPTH Framework helps you avoid common mistakes, build lasting credibility, and support long-term behavior change.
Want more expert insights? Consider registering for the PharmChek® Academy, where you’ll gain access to conversations with colleagues, peers, and industry experts around specific topics relevant to your success with the PharmChek® Sweat Patch.